Medical professionals often view nail polish as something that should be avoided.
This is not only because staff wearing nail polish may leave a negative impression, but also because a patient’s nail polish can sometimes interfere with medical care.
However, the situation can be a little different in elderly care settings.
In Japan, “welfare nail care” (fukushi nail)—nail services provided to older adults and people living with illness or disability—has been gaining attention.
There is even a term, “beauty care in eldercare” (kaigo beauty), reflecting a growing movement to integrate beauty into care.
In this article, I will focus on Aqure Nail Pen, a nail product developed specifically for welfare and eldercare settings that I learned about at an exhibition last year.
A product described as “designed for care settings” may sound appealing.
But in practice, introducing something new into a facility is much easier when you can clearly explain what is beneficial, and why, with sound reasoning.
That’s why this article takes a deeper look at Aqure Nail Pen—from the chemistry behind nail products to how those mechanisms may translate into practical advantages in care environments.
Disclaimer: This article is based on the author’s personal views and research.
It does not guarantee accuracy or completeness.
Any decision to purchase or use the product introduced here should be made at your own discretion and responsibility.
This article may contain affiliate links (PR).
Nail Products in Elderly Care Settings
If you’ve ever worn nail polish, you probably know the feeling: seeing neatly polished nails can lift your mood.
One reason nail services are introduced in elderly care settings is precisely that—supporting positive emotions and self-image.
Below, I summarize the benefits of using nail products in care settings and the key points to watch.
Benefits
● Improves mood:
Beautiful fingertips can help people feel more positive, and sometimes even soften facial expressions.
● Creates conversation:
It can become a natural conversation starter with staff and may even encourage interaction among residents.
● Encourages hand activity:
Choosing colors and applying polish can provide an opportunity to use and stimulate the fingers and hands.
Points to Watch (Risks)
● Odor:
Some people are sensitive to the strong, solvent-like smell associated with nail products.
In poorly ventilated spaces, odor can become a burden.
● Waiting time for drying:
In time-limited settings, not being able to wait until nails dry can be a reason the activity doesn’t go smoothly.
● Effort required for removal:
Typical nail polish often requires remover, which can add preparation/cleanup work and cause discomfort due to odor or irritation.
● Difficulty applying:
If staff are not used to applying nail polish—or if the person receiving care has limited hand movement or cognitive decline—application may be difficult.
● Impact on observation and monitoring:
Polished nails can make nail color harder to check and may affect SpO₂ measurement.
● Infection-related concerns:
If someone has nail conditions such as onychomycosis (a fungal nail infection), extra hygiene considerations are needed when products are shared.
The Chemistry of Typical Nail Polish
“Chemistry of nail polish” may sound complicated, but the core mechanism is simple.
A polymer-containing liquid is applied to the nail, and as it dries, a thin film remains on the nail surface.
The odor, drying time, and ease of removal are often determined less by the polymer itself and more by the other ingredients blended into the formulation.
Let’s look at typical components, with the chemistry explained in simple terms.
Film-Forming Agents (Resins/Polymers)
A “resin” in this context is a polymer—large molecules formed by chains of repeating units.
Resins may sound unfamiliar, but plastics are also resins (synthetic polymers), so it’s more familiar than it sounds.
With nail polish, the polymer remains as a thin film once the liquid dries.
A common film-forming polymer used in many conventional nail polishes is nitrocellulose.
Nitrocellulose is a polymer derived from plant-based cellulose, in which some of the -OH groups are replaced with nitrate ester groups (-O–NO₂).
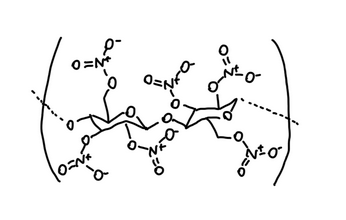
Nitrocellulose is known for being highly flammable.
However, nitrocellulose alone may lack sufficient adhesion and gloss, so it is often combined with other resins.
Plasticizers
Plasticizers insert themselves between polymer chains and increase mobility, making the film more flexible.
A nitrocellulose film can be hard and prone to cracking, so plasticizers are commonly used.
Volatile Solvents
Polymers are large molecules; without solvents, the product would not have a workable liquid form for application.
Solvents are therefore added to adjust viscosity and improve spreadability.
Key requirements for these solvents are:
- They must mix well with the polymer, and
- They must evaporate quickly after application.
A commonly used group of solvents in nail polish is ester solvents, such as ethyl acetate.
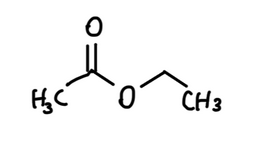
Ester solvents are often chosen because they mix well with film-forming polymers and evaporate quickly, helping the polish dry faster.
This is influenced by chemical structure.
Ester solvents contain the functional group –COO–, and the C=O (carbonyl) portion has an electrical polarity (a partial charge distribution).
Molecules are formed by atoms sharing electrons, but within a molecule, electrons are not always shared equally.
Between oxygen (O) and carbon (C), oxygen pulls electrons more strongly.
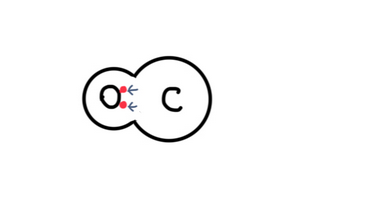
As a result, the oxygen side becomes partially negative, while the carbon side becomes partially positive.
Similarly, in nitrocellulose, the –O–NO₂ region also has a polarized structure.
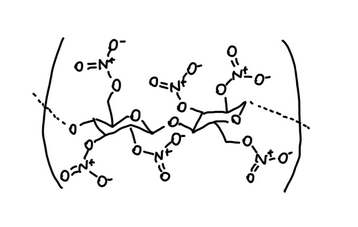
Because polarized regions can attract each other, ester solvents and nitrocellulose tend to mix well and can form a stable solution at the molecular level.
However, this attraction is not extremely strong.
For example, molecules that contain an –OH group (such as water and alcohols) can form hydrogen bonds.
Oxygen pulls electrons strongly, making the hydrogen nearby partially positive; that hydrogen is then attracted to a partially negative site (like oxygen) on another molecule, forming a relatively strong intermolecular interaction.
Hydrogen bonding makes molecules hold together more tightly, making them less likely to escape into the air.
Ester molecules typically do not contain –OH, so they form hydrogen bonds less easily and tend not to clump together as strongly.
As a result, the solvent still works well with the polymer but is not bound too tightly—so it can evaporate readily after application, leaving the polymer behind as a thin film on the nail.
These volatile solvents are also the main source of the sharp smell associated with conventional nail polish.
Because the polymer–solvent interaction is not excessively strong, solvent molecules can rapidly escape into the air, making the odor feel more intense right after application.
Other Components (Pigments, Pearlescent Materials, Gelling Agents, etc.)
Pigments and pearlescent particles tend to settle over time, so gelling agents and related additives may be used to reduce settling.
Aqure Nail Pen
What Is Aqure?
Aqure Nail Pen is a nail product sold by Lavina Co., Ltd., developed for welfare and eldercare settings.
In addition to selling the nail pen, the company provides beauty recreation programs at welfare facilities and offers training seminars related to beauty in care.
Their message appears to emphasize the idea that “no matter your age, and even in a facility, it should be normal to enjoy beauty.”
On their website, Aqure is introduced as a “pen-type, water-based manicure whose main component is water, designed to be safe and reassuring.”

To summarize the product features briefly:
- A water-based nail product designed for welfare settings, with minimal sharp odor and relatively fast drying.
- A thicker, drip-resistant texture, yet it spreads smoothly during application.
- Can be removed by peeling (no nail polish remover needed), reducing preparation and cleanup burden.
- Advertised as compatible with SpO₂ measurement.
So, what exactly makes it “safe and reassuring”?
Because “safe” can mean different things depending on context, I will focus on a practical viewpoint: how Aqure reduces factors that commonly become burdens in care settings, and I will explain that based on ingredients and chemistry.
Why It Fits Care Settings
Low Odor
As explained earlier, the characteristic smell of conventional nail polish mainly comes from volatile solvents that rapidly evaporate after application.
Aqure uses film materials that can be handled in water-based systems (such as polyurethane), which helps avoid relying primarily on ester solvents that tend to cause strong odor.
That said, polyurethane is not “dissolved in water” in the same way that small molecules dissolve.
Polyurethane contains long polymer chains with water-repellent segments, and polymer chains can stick together through interactions such as hydrogen bonding.
Overall, polyurethane tends to be poorly water-soluble.
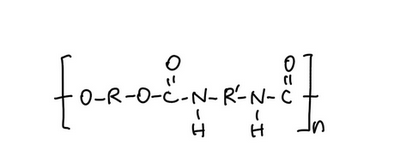
In water-based formulations like Aqure, the polymer is typically not dissolved; rather, it is present as a dispersion—tiny polymer particles suspended throughout water.
Common strategies to keep polymer particles dispersed in water include:
- giving the particle surface an electrical charge so particles repel each other, and/or
- surrounding particles with surfactant-like components that stabilize the dispersion.
Based on the ingredient list, Aqure likely uses one or both of these approaches.
After application, as water evaporates, dispersed polymer particles move closer together, pack more densely, and then merge into a continuous film.
Because the film forms through water evaporation rather than rapid evaporation of strong-smelling ester solvents, Aqure tends to have less sharp odor.
Easy to Apply
Because Aqure is water-based, the liquid would normally be more runny and prone to dripping or streaks, making application harder.
To improve handling, it is likely thickened using polyacrylic acid and stearalkonium hectorite.
● Polyacrylic acid
Polyacrylic acid is a polymer that contains many carboxylic acid groups (-COOH).
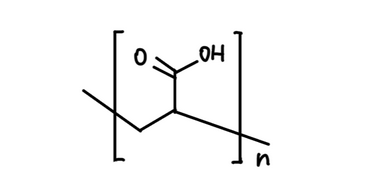
Depending on pH, these -COOH groups can lose H⁺ and become carboxylate (-COO⁻), increasing negative charge along the polymer chain.
As negative charges increase, polymer chains repel each other and expand.
In that expanded state, they hold more water, causing the polymer network to swell.
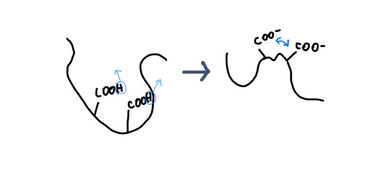
This swelling restricts water movement and increases viscosity, creating a thicker, more manageable texture.
● Stearalkonium hectorite
Hectorite is a plate-like clay mineral.
During crystal formation, some sites in the clay layers may undergo isomorphic substitution (where one ion in the crystal lattice is replaced by another of similar size).
For example, some Mg²⁺ sites may be replaced by monovalent cations such as Li⁺.
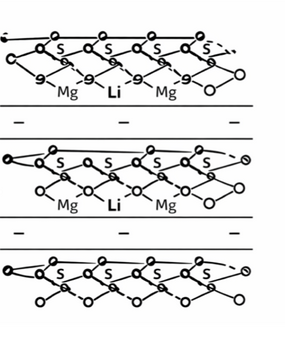
This reduces the overall positive charge in the layer, leaving the clay layers net negatively charged.
To balance this negative charge, cations such as Na⁺ occupy the interlayer spaces.
When a quaternary ammonium cation such as stearalkonium is introduced, it can exchange with interlayer Na⁺, producing an organomodified clay—meaning the clay surface becomes more compatible with organic components.
This material is called stearalkonium hectorite.
Stearalkonium hectorite particles are plate-like, and they tend to have charge characteristics such as: the flat faces being relatively negative, and the edges being relatively positive.
Because of this, the face of one platelet can electrostatically attract the edge of another, forming a three-dimensional card-house structure.
That structure restricts movement in the liquid and increases viscosity.
However, the interactions are not extremely strong. Under shear (rubbing/spreading during application), the structure temporarily breaks down and the liquid becomes more fluid, making it easier to spread.
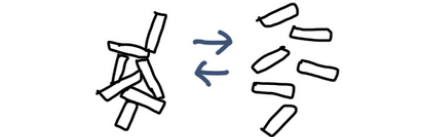
This behavior is called thixotropy—a useful property that helps a product resist dripping yet spread smoothly when applied.
Dries Quickly
Aqure is described as drying in roughly 30 seconds to 1 minute, but water is not as volatile as ester solvents.
One reason Aqure may still feel like it dries quickly is that thixotropic behavior helps the product spread thinly and evenly, reducing thick application.
A thinner film naturally feels faster to dry.
Can Be Peeled Off
Aqure is likely easier to peel because the film forms differently from conventional nail polish.
Conventional nail polish typically uses a polymer dissolved in an organic solvent.
When a polymer is “dissolved,” it does not remain as particles; the polymer chains are dispersed uniformly at the molecular level within the solvent.
When applied to the nail, the polymer solution can flow into tiny surface irregularities.
After that, the solvent evaporates, leaving the polymer to solidify in place as a film.
Because the polymer penetrates fine unevenness and solidifies there, removal often requires solvents (remover) to dissolve the film again.
In contrast, Aqure uses a water-based dispersion of polymers such as polyurethane.
Because the polymer is not dissolved molecularly in the same way, the system is closer to polymer particles sitting on the nail surface while water serves as the dispersing medium.
As water evaporates, the particles draw closer, pack together, and eventually merge into a continuous film.
In this mechanism, the film tends to form as a more unified layer while bonding less strongly into microscopic surface irregularities—making it more likely to be removed by peeling.
As an image, it is similar to the difference between: liquid glue that seeps into fine gaps and hardens, and a sticker that sits on the surface as one sheet.
This may explain why Aqure can often be removed without nail polish remover.
Allows SpO₂ Measurement
Aqure is marketed as compatible with SpO₂ measurement using a pulse oximeter.
The exact reason is not fully clear, but it is likely related to the film being designed not to interfere significantly with the light used by pulse oximeters.
SpO₂ refers to peripheral oxygen saturation—the percentage of hemoglobin carrying oxygen, estimated noninvasively.
In clinical practice, it is measured routinely, like other vital signs such as body temperature and blood pressure.
SpO₂ values generally correlate closely with SaO₂ (arterial oxygen saturation) measured from arterial blood, but SpO₂ is far easier to obtain.
A pulse oximeter is commonly clipped onto a fingertip.
It shines light through tissue and estimates oxygen saturation by analyzing how the detected light changes with pulsatile blood flow.
When nails are polished, film thickness and pigments can alter light transmission, sometimes preventing accurate measurement.
Aqure is designed to spread smoothly, making thick, uneven layers less likely.
If the film remains thin and even, it may be less disruptive to the pulse oximeter’s optical path, reducing measurement interference.
Where to Buy
Aqure Nail Pen is available not only through the official website and welfare product online shops, but also on Rakuten and Amazon in Japan.

There are various colors, including clear and glitter options.
Considering that nail products can be purchased at 100-yen shops, JPY 1,870 per pen (official site price as of January 2026) is not a casual purchase.
For that reason, being able to purchase a single pen as a trial before introducing it into a facility is helpful.
My Experience and Practical Notes
I bought a color I liked as a trial and used it myself.
The following are personal impressions and practical notes, organized by topic.
Odor
Before application and while drying, I hardly noticed the sharp, typical nail-polish smell.
I personally dislike that smell strongly, so this point alone felt like a meaningful advantage for use in care settings.
Ease of Application
The liquid inside was quite thick—tilting the pen didn’t make it move easily.
When I dispensed a larger amount, it held its shape in a small mound.
However, on the nail it spread well and felt like it became smoother and more fluid as I moved it across the surface.
Because it is pen-shaped, you can hold it like a pencil, and the tip is a firm, flat brush.
Even though I am not used to applying nail products, I found it easy to use.
Drying Time
It genuinely felt like it dried quickly, so I think it could be practical even for activity sessions with limited time.
SpO₂ Measurement
Using an inexpensive pulse oximeter I had on hand, I was able to measure SpO₂ on a finger after applying the product.
That said, results may vary depending on the device and the way the product is applied.
Also, once nails are coated, observing nail color becomes more difficult.
Therefore, extra caution is needed for individuals who require close medical observation or who have a higher risk of sudden decline.
In places like day services, where many users are relatively stable, it may not be necessary to be overly strict.
Still, older adults can change condition quickly.
A practical approach may be to leave one fingernail uncoated for observation.
For individuals for whom SpO₂ is a critical monitoring item, it may be safer not to use nail products at all.
I recommend following facility policies and medical staff guidance.
Peeling Off
After drying, I could lift an edge and peel it off easily.
Sometimes it came off cleanly as one sheet, but if the layer was too thin or uneven, it broke into small flakes.
On one occasion, it came off during bathing without me noticing.
For welfare nail care, easy removability is generally a benefit.
However, for individuals with pica or a tendency to put non-food items in the mouth, peeled fragments may create a risk of ingestion or aspiration.
Suitability should be considered based on the person’s condition.
Hygiene
From an infection-control perspective, assigning one pen per person would be ideal—but that is often unrealistic for facility recreation.
Aqure works like a regular pen: liquid continuously wicks into the brush tip.
Because of that structure, even after wiping the tip, product may remain within the brush.
When I wiped the brush thoroughly with an alcohol pad, I was able to remove a noticeable amount.
If the product is shared, this kind of hygiene step may improve safety.
If someone has any nail abnormalities, it is best not to decide on your own—avoid use and consult a healthcare professional.
Closing Thoughts
As people live longer and quality of life—purpose, identity, “living as oneself”—becomes more important, I believe incorporating beauty into care settings can be meaningful.
When I tried nail care again after a long time, I personally felt a little brighter.
Creating smiles through recreational activities is one of the joys and rewards of eldercare.
Aqure appears to include design choices that reduce common barriers to using nail products in care settings.
Depending on facility rules, it may be worth considering as one option for recreational programs.
I also share information based on my perspective as a nurse, combined with experience reading and interpreting patents.
I provide explanations of medical and care products, write technical articles, and support market entry and expansion.
If you are interested, feel free to contact me through the form on my blog.
Disclaimer: This article is provided for reference only and does not guarantee accuracy.
Any purchase or use of products is at your own discretion and responsibility.
References
- Raveena Inc. (Lavina Co., Ltd.)
https://raveena.jp - The Society of Cosmetic Chemists of Japan (SCCJ)
https://www.sccj-ifscc.com/ - Japan Health and Welfare Nailist Association (JHWN) (Japanese: 日本保健福祉ネイリスト協会)
https://fukushinail.jp/ - McMurry, John. McMurry Organic Chemistry, 8th ed. (Japanese edition). Tokyo: Tokyo Kagaku Dojin, 2012.
- Urabe, Yoshinobu. Kagaku no Shin Kenkyu [New Study of Chemistry]. Tokyo: Sanseido, 2019.

