This article presents a case involving a patient who was taking calcium polystyrene sulfonate (generic name, hereafter referred to as Ca-type ion exchange resin), marketed in Japan under the brand name Kalimate, for the treatment of hyperkalemia.
The patient was in a nearly bedridden condition, and after starting oral administration of Kalimate, developed extremely hard stools and severe difficulty with defecation.
Constipation has been reported as a side effect of this medication, but in this case the symptoms were particularly severe. (This is an individual example, and the severity of symptoms varies among patients.)
Using this case as a starting point, I would like to review the mechanism of action of the drug and organize the possible factors that may influence stool consistency.
Note: This article is based on the author’s personal research and perspective. It does not guarantee accuracy or completeness, and it is not intended to promote or recommend any specific product. Use of this content should be at the reader’s own discretion and responsibility.
The English version of this article is currently under preparation.
Hyperkalemia
What is potassium?
Potassium (K) is an alkali metal belonging to Group 1 of the periodic table.
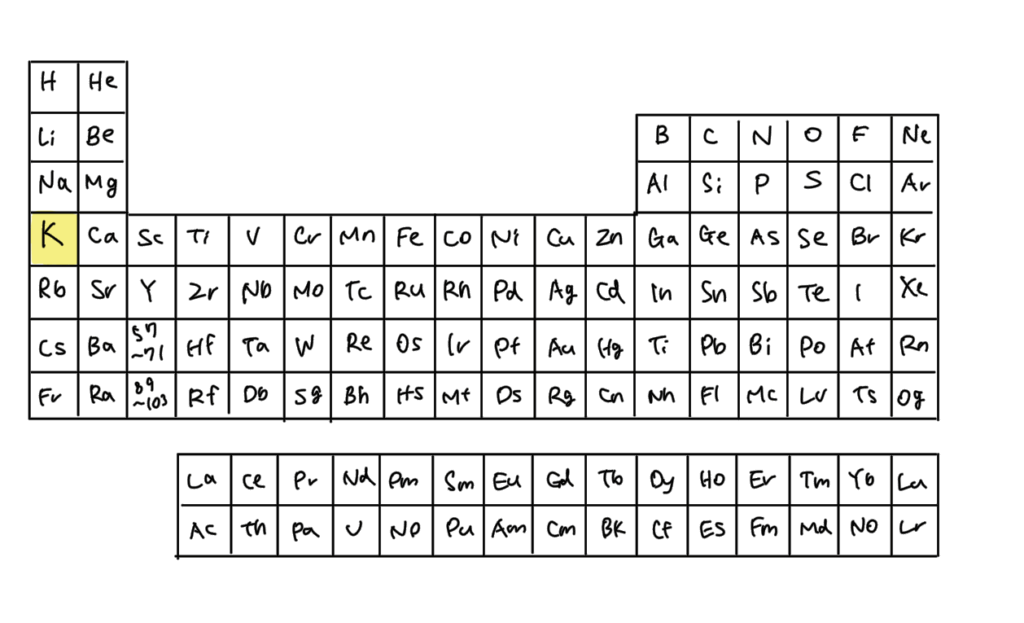
It has a single outermost electron, which it readily loses to achieve a stable closed-shell structure, making it prone to becoming a cation.
Sodium (Na), another alkali metal, is familiar as the main component of table salt.
In the human body, potassium exists as K⁺, is abundant in intracellular fluid, and balances with sodium ions (Na⁺), which are predominant in extracellular fluid.
Together, these ions maintain normal cellular function.
Definition of hyperkalemia
Hyperkalemia is generally defined as a serum potassium concentration above the normal range of approximately 3.5–5.0 mEq/L. (Exact thresholds may vary slightly across references and institutions.)
mEq (milliequivalent) is not a unit of weight like milligrams. It reflects the ionic charge, allowing comparison of electrolytes with different atomic weights and valences.
It indicates the physiological impact of electrolytes in ongoing biochemical reactions.
Causes and symptoms
The most common cause of hyperkalemia is renal failure.
The kidneys normally filter blood, excreting waste products and excess electrolytes to maintain homeostasis.
When kidney function declines, K⁺ excretion is impaired, and serum levels rise.
Severe hyperkalemia increases the risk of arrhythmia and cardiac arrest.
Therefore, strict control of potassium intake is essential in patients with renal impairment.
Medication errors involving potassium formulations have also been reported, and many hospitals establish internal rules requiring nurses and other staff to cross-check dosing instructions to prevent such accidents.
Relationship between hyperkalemia and cardiac function
Membrane potential and action potential
Why does hyperkalemia lead to arrhythmias or cardiac arrest?
The answer lies at the level of nerve and muscle cell physiology.
Nerves function as information pathways, transmitting sensory input, motor commands, and autonomic regulation via electrical signals.
These signals arise from the movement of ions.
In neurons, changes in electrical charge generate nerve impulses, which is transmitted to subsequent cells to coordinate body functions.
Normally, intracellular fluid contains a higher concentration of K⁺, while extracellular fluid has more Na⁺.
This distribution is maintained by the sodium-potassium pump, which actively transports Na⁺ out and K⁺ in.
In addition, K⁺ tends to flow outward through leak channels, leaving the intracellular environment more negative than the outside.
This electrical difference is called the membrane potential. A resting state is referred to as polarization.
To trigger cardiac contraction, cells must undergo excitation. This occurs through the following sequence:
- Na⁺ permeability increases
- Na⁺ rapidly flows into the cell, making the membrane potential temporarily positive (depolarization)
- K⁺ then flows out, restoring the negative potential (repolarization)
This rapid change in membrane potential is called an action potential.
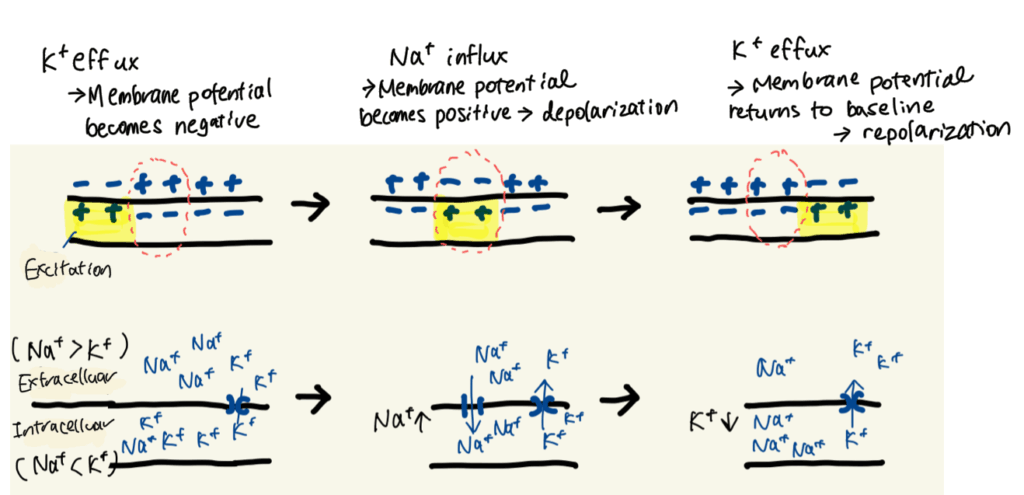
It is the fundamental mechanism by which nerve signals are transmitted and cardiac muscle contraction is regulated.
In the case of hyperkalemia
When extracellular K⁺ concentration rises, the efflux of K⁺ decreases, and the resting membrane potential shifts toward a more positive value.
As the electrical gradient diminishes, Na⁺ influx during depolarization is reduced.
This slows cellular excitation, weakens cardiac contraction, and increases the risk of arrhythmia or cardiac arrest.
Mechanism of action of Kalimate

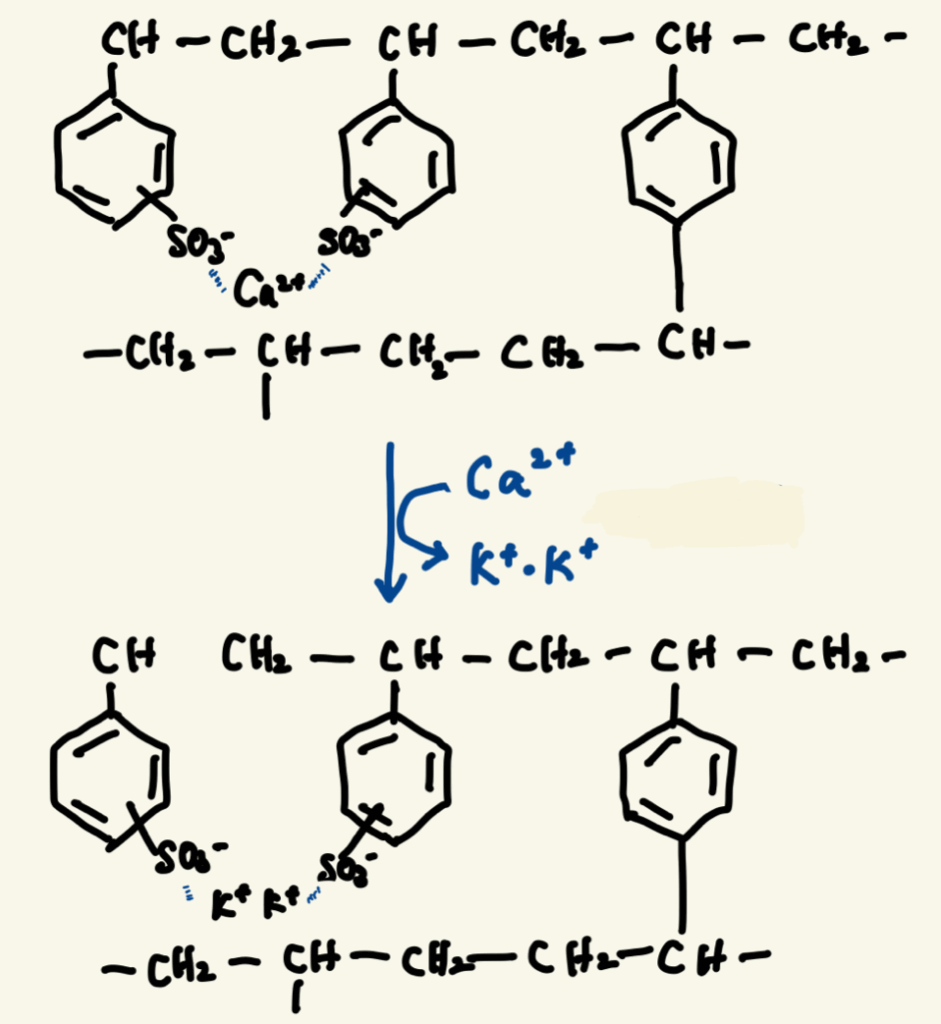
Kalimate is composed of calcium polystyrene sulfonate, classified as a cation exchange resin.
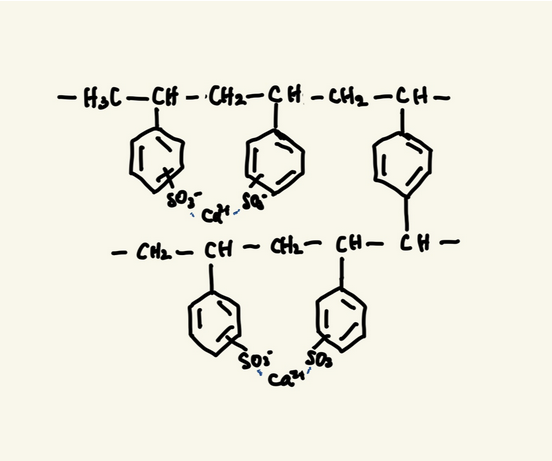
This resin consists of polystyrene chains with sulfonic acid groups (–SO₃⁻), cross-linked to form a network structure. The sulfonic acid groups are bound to Ca²⁺.
When the resin reaches the intestine, the Ca²⁺ is exchanged for K⁺, binding potassium in the gut and promoting its excretion in the stool. This helps reduce serum potassium levels.
A related resin, sodium polystyrene sulfonate (Kayexalate), uses Na⁺ instead of Ca²⁺.
While it also removes potassium via ion exchange, the released Na⁺ can be absorbed and may influence cardiovascular function, such as contributing to hypertension.
Mechanism of constipation
Why does constipation occur with Kalimate?
The polystyrene backbone is hydrophobic, so when mixed with stool, it resists water absorption.
As a result, the stool becomes drier and harder to pass.
Although sulfonic acid groups interact with water, the polymer matrix is rigid and cannot retain water effectively.
Clinically, Kalimate is suspended before administration, but when mixed with water it tends to precipitate, resembling starch sedimentation.
In the presented case, a relatively high dosage likely compounded the problem, causing the stool to become excessively hard.
In contrast, sodium polystyrene sulfonate releases Na⁺, which can increase intestinal osmotic pressure and help retain water in the stool.
In theory, this may contribute to softer stool consistency.
However, clinical evidence remains limited, and constipation can still occur with sodium-based resins.
Related patents
Patent publication: JP 2004-149525 (P2004-149525A)
Publication date: May 27, 2004
Title: Potassium ion adsorbent, its production method, and therapeutic agent for hyperkalemia
Limitations of conventional treatments
<acrylic resin>
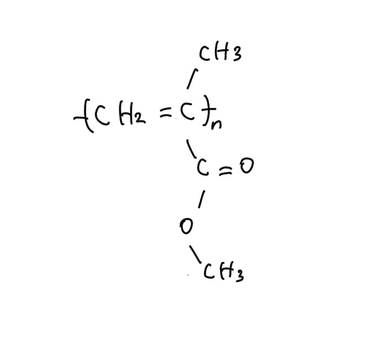
- Acrylic-based ion exchange resins
- Hydrophilic functional groups give high swelling capacity
- Carboxyl groups (–COOH) exchange H⁺ for K⁺
- Advantage: swelling increases surface area, improving contact with K⁺
- Limitation: weaker binding affinity compared to sulfonic acid groups
- Polystyrene sulfonate resins (e.g., Kalimate)
- Strong electrostatic binding of K⁺ via sulfonic acid groups
- Limitation: hydrophobic, low swelling, smaller contact area, requiring larger doses for effect
The present invention
To address these issues, the patent describes polymerizing sulfonated vinyl monomers with water-soluble cross-linking monomers in an aqueous solvent.
The resulting polymer maintains strong K⁺ adsorption capacity while gaining hydrophilicity and swelling ability. This design increases surface area and enhances efficiency of potassium removal.
Compared with conventional calcium polystyrene sulfonate, the new resin aims to achieve adequate potassium binding at lower doses.
Closing thoughts
Kalimate, composed of calcium polystyrene sulfonate, is effective in potassium binding but is hydrophobic and poorly swollen, which can contribute to stool hardening and constipation.
The patented hydrophilic polymer design offers a potential approach to improve adsorption efficiency while reducing the risk of constipation.
Through this case, reviewing both pharmacological mechanisms and related patents highlights potential directions for developing more user-friendly therapeutic options.
Currently, I apply my background as a nurse and my knowledge of patent technologies to publish explanatory articles on medical materials and care products, as well as to support market entry initiatives. If you are interested, please feel free to contact me through the blog form.
Disclaimer: This article is provided for informational purposes only. It does not guarantee accuracy or completeness. It is not intended to advertise, recommend, or promote any specific product. Please use the information responsibly and at your own discretion.
References
- JP Patent Publication No. 2004-149525, Potassium ion adsorbent, its production method, and therapeutic agent for hyperkalemia, May 27, 2004.
- Guyton AC, Hall JE. Textbook of Medical Physiology, 11th ed. Elsevier Japan, 2010.
- Urabe Y. Shin Kenkyu Kagaku (New Studies in Chemistry), Sanseido, 2010.
- Ministry of Health, Labour and Welfare, Japan. https://www.mhlw.go.jp/topics/2006/11/dl/tp1122-1e19.pdf