Intermittent catheterization is a representative medical procedure for patients who experience difficulty with urination.
However, it often causes pain or discomfort and must be performed multiple times a day, which can be a considerable burden for patients.
In this article, I will focus on a patent related to urethral catheters, discussing common challenges during catheterization and design innovations that aim to address them.
Disclaimer: This article is based on my own research and professional perspective. It does not guarantee the accuracy of the information provided. Decisions regarding purchase or use of products should be made at your own responsibility. Some links in this article may contain affiliate programs (PR).
What is a Urethral Catheter?
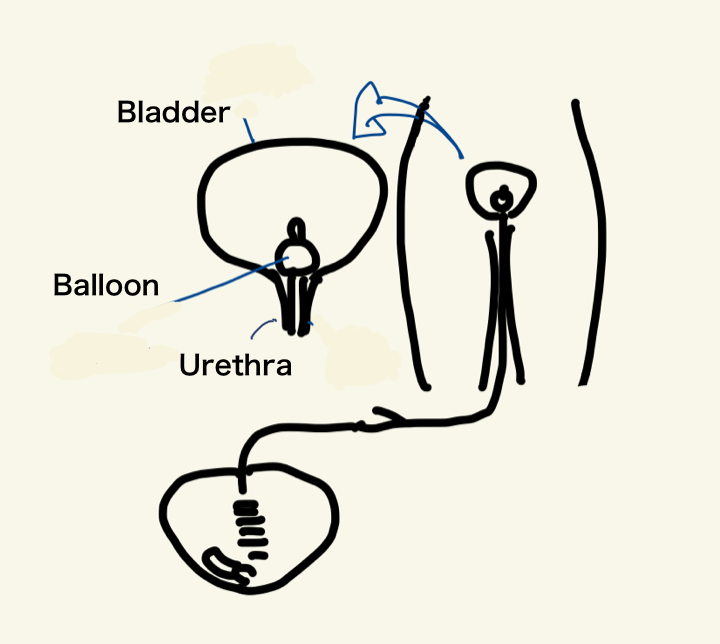
A urethral catheter is a medical device inserted through the urethra into the bladder to drain urine outside the body.
The act of draining urine through a catheter is called catheterization.
It is commonly required in cases of temporary urinary retention after surgery or illness, or in patients with chronic urinary retention (urinary retention refers to a state where urine accumulates in the bladder but cannot be voided).
There are two major types of catheters:
- Intermittent catheters
Inserted only when needed and removed immediately after drainage.
Note: The above products are affiliate links for the Japanese market (Amazon Japan, Rakuten).
- Indwelling (Foley) catheters
Fixed in place inside the bladder by inflating a balloon at the tip, allowing continuous drainage for a certain period.
Note: The above products are affiliate links for the Japanese market (Amazon Japan, Rakuten).
This article focuses on intermittent catheters.
Because they are used frequently and often for self-catheterization, minimizing discomfort and ensuring safety are of utmost importance.
Patent on Urethral Catheters
- Publication No.: JP 2024-102271 A
- Publication Date: July 30, 2024
- Title of Invention: Urethral Catheter
Challenges in Catheterization
The following issues are frequently encountered during catheterization:
Pain and Discomfort
Since the catheter is inserted into a narrow urethra without anesthesia, pain and discomfort are common.
Friction with the urethral mucosa or entrapment of tissue in the drainage orifice can be the cause.
Risk of Tissue Injury
During insertion and removal, the mucosa may be abraded.
In male patients, prostatic enlargement can make insertion difficult, and forced advancement may result in bleeding.
This is a point I pay close attention to in practice.
At the tip of the urethral catheter, there are drainage orifices, through which urine from the bladder enters the catheter and is drained outside the body.
If one of these orifices becomes suddenly occluded, the pressure inside the catheter drops sharply, generating a negative pressure pulse.
This suction effect can draw the bladder wall into the orifice, potentially resulting in mucosal injury.
Urinary Tract Infection (UTI)
The urinary tract is normally sterile.
However, residual urine can allow bacteria to proliferate, increasing the risk of UTI.
Bacteria may also be introduced into the urethra during catheter insertion, and despite aseptic technique, it is nearly impossible to maintain a completely sterile environment.
Technical Solutions to These Challenges
The patent proposes several structural innovations to reduce these risks.
Minimizing Tissue Damage
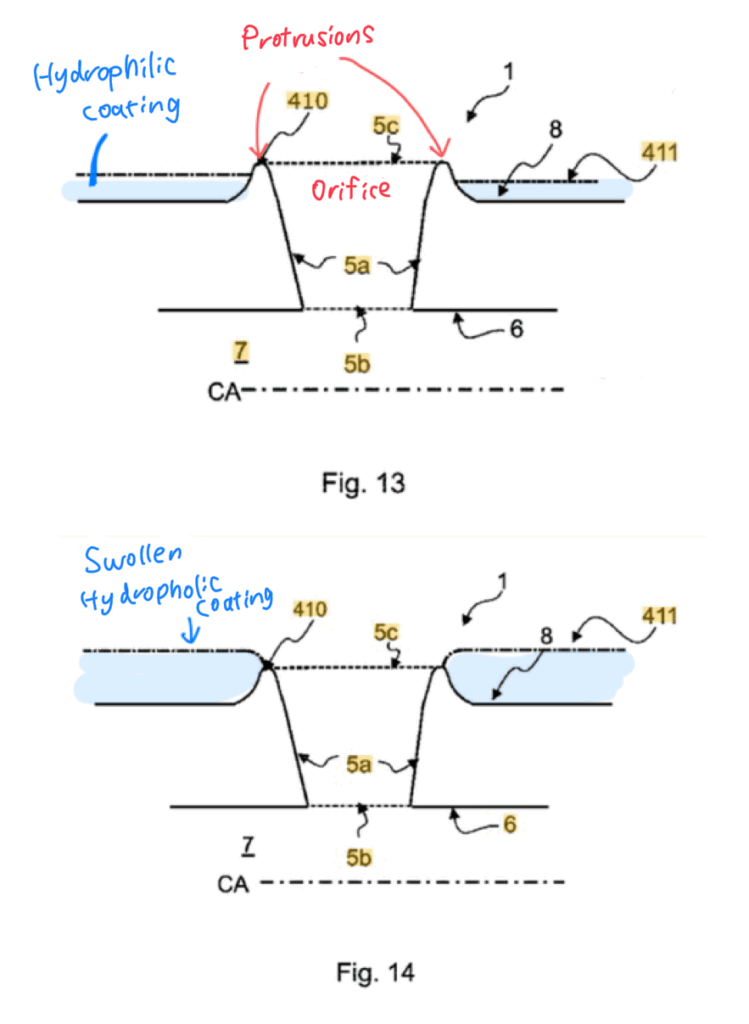
- Protrusions around the drainage orifice
There are protrusions around the drainage orifice, designed so that the urethral wall is lifted during catheter insertion. This prevents tissue from being drawn into the orifice and reduces the risk of mucosal abrasion.
(Figure cited from JP Patent Application 2024-102271, with annotations added for explanation.)
- Hydrophilic coating on the catheter surface
The coating absorbs water and swells, covering the protrusions and creating a smoother surface.
This reduces resistance during withdrawal and minimizes mucosal injury.
However, since this is designed for intermittent catheterization, prolonged indwelling (5–20 minutes, as described in the patent) may increase adhesion to the mucosa, potentially raising the risk of injury upon removal.
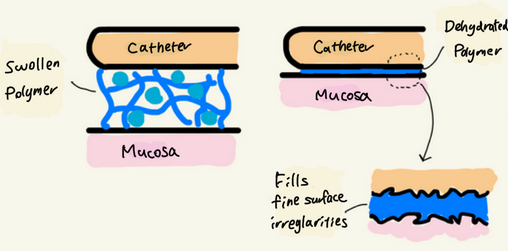
- Properties of hydrophilic polymers
Hydrophilic polymers form a network structure that absorbs water, swelling into a gel that facilitates lubrication.
When hydration decreases, the polymer fills microscopic surface irregularities, increasing contact area and enhancing van der Waals forces.
This can result in stronger adhesion between the catheter and mucosa.
Thus, polymers that initially aid lubrication may exhibit adhesive characteristics over time.
- Smaller, multiple drainage orifices
The use of many small openings reduces the likelihood that all orifices will become blocked simultaneously.
Even if occlusion occurs, the reduced orifice size weakens the suction force, lowering the risk of bladder wall injury due to negative pressure pulses.
Ensuring Smooth Flow and Reducing Residual Urine
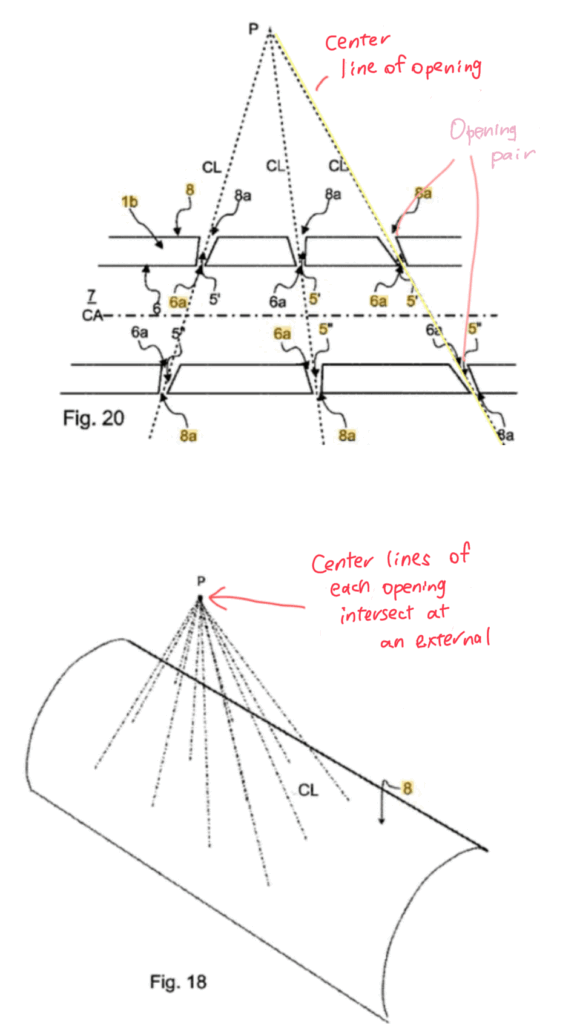
- Strategic arrangement of orifices
The centerlines of the orifices are extended into the bladder such that at least two lines intersect at one point.
This allows urine to flow directly into each orifice without interference between flow streams, reducing turbulence and energy loss.
- Paired orifices
Orifices are arranged in pairs along the same centerline.
If one side becomes occluded by the urethral wall, the opposite orifice remains open, preventing complete blockage.
(Figure cited from JP Patent Application 2024-102271, with annotations added for explanation.)
- Cross-sectional design
The combined cross-sectional area of the drainage orifices is designed to be equal to or larger than that of the catheter lumen, ensuring adequate flow volume.
Closing Thoughts
The catheter described in this patent was filed in Denmark. I have not seen it in actual practice, but based on its structure, it seems practical and user-friendly.
Such innovations in overseas medical devices spark my interest, and if given the opportunity, I would like to compare them with the products currently in use.
Drawing from both my nursing experience and my knowledge of patents, I will continue to share insights into medical devices and care products, while also contributing to the dissemination of technical information and market development support.
Please feel free to contact me via the blog’s form if you are interested.
Disclaimer: This article is provided for informational purposes only and does not guarantee accuracy.
Product purchases or use should be made at your own judgment and responsibility.
References
- Beyar Kim, Seiar Beamte Timbak Flezlek. Urethral Catheter. JP 2024-102271 A, published July 30, 2024.
- Toyohiro Okaniwa. Byoki ga Mieru Vol.8: Kidney & Urinary System, 3rd Edition. Medic Media, 2019.
- Namisuke Kubota. Tokoton Yasashii Ryutairikigaku no Hon (Introduction to Fluid Dynamics). Nikkan Kogyo Shimbun, 2007.


コメント